The immunological interplay between vaccination and the intestinal ... - Nature.com
Vaccination is the most cost-effective life-saving medical intervention1. However, substantial variation in individual immune responses to vaccination exists2. Lower vaccine immunogenicity, especially to oral vaccines, is often reported in developing countries3,4,5. Many intrinsic and extrinsic factors, such as age, genetics, pre-existing immunity, nutritional status and comorbidities contribute to the variations in vaccine responses6.
Growing evidence shows that the composition of the intestinal microbiota, which is known to play an important role in the development and regulation of immune responses7, influences responses to vaccination8,9,10. Studies which have investigated this have shown that a higher relative abundance of the phylum Actinobacteria is consistently associated with higher vaccine responses and a higher relative abundance of Bacteroides with lower responses, while the association between the relative abundance of the phyla Firmicutes and Proteobacteria and vaccine responses varies for different genera and species11,12,13,14,15,16,17,18. Almost all of these studies investigated the association for oral vaccination11,12,14,15,16,17,18, only three studies investigated responses after intramuscular vaccination13,19,20.
Only a few studies have investigated whether the administration of vaccines is associated with changes in the composition of the intestinal microbiota12,20,21,22,23,24,25,26, and whether the changes induced by vaccination, in turn, influence vaccine immunogenicity20. These studies have reported inconsistent results. In humans, the administration of oral typhoid or an intranodal and intramuscularly human immunodeficiency virus (HIV)-1 vaccine was not reported to significantly affect the composition of the intestinal microbiota one week to several months after vaccination12,21,22. The first two studies used amplicon sequencing, while the third used shotgun metagenomic sequencing. A recent study using metagenomic sequencing, reported that the intramuscular administration of an inactivated and an mRNA SARS-CoV-2 vaccine was associated with lower bacterial diversity, an increase in the relative abundance of Bacteroides caccae and a decrease in the relative abundance of Clostridiales (Coprococcus comes, Dorea longicatena and Ruminococcus obeum) in the intestinal microbiota one month after vaccination20. Animal studies have also shown that the administration of vaccines can lead to changes in the intestinal microbiota: in mice, an increase in the relative abundance of Bacteroides has been reported after intramuscular vaccination with a Mycobacterium tuberculosis vaccine25 and compared with mice vaccinated with a placebo, mice vaccinated intramuscularly with an HIV T-cell immunogen had a higher relative abundance of Clostridiales (Eubacterium xylanophilum, Roseburia and Ruminococcus)26. In rhesus macaques, the intradermal administration of a combined HIV-1 DNA/protein vaccine has been shown to lead to an increase in the Firmicutes/Bacteroidetes ratio and a decreased relative abundance of Prevotella, Alloprevotella, Bacteroides, Acetobacteroides, Falsiporphyromonas and Anaerocella. Interestingly, the abundance of Prevotella negatively correlated with rectal HIV-1 specific immunoglobulin G levels24. In piglets, the administration of an oral Lawsonia interacellularis vaccine led to an increased relative abundance of Streptococcus and Ruminococcus, and a decreased relative abundance of Clostridium23.
The proposed mechanisms behind the influence of the composition of the intestinal microbiota on vaccine immunogenicity include cross-reactive epitopes between microbes and vaccine antigens, modulation of B cell responses through microbial metabolites such as short-chain fatty acids (SCFAs), and the provision of natural adjuvants through certain microbes (Fig. 1)10. The latter includes the stimulation of pattern recognition receptors through bacterial components, such as the stimulation of toll-like receptors (TLR)-5 through flagellin27 or nucleotide-binding oligomerization domain (NOD)-2 receptors through muramyl dipeptide which is part of peptidoglycan28. A recent study shows that "silent" flagellins, which are weak TLR-5 agonists are abundant in Lachnospiraceae in the intestinal microbiota and overrepresented in non-industrialised populations29. The microbiota has also been shown to regulate type I interferon production by plasmacytoid dendritic cells, which stimulates conventional dendritic cells to more efficiently prime antigen-specific T cell responses30. Moreover, in mice, SCFAs (acetate, propionate and butyrate) produced by microbes of the intestinal microbiota through fermentation of dietary fibres have been shown to enhance B cell metabolism and regulate gene expression to promote B cell differentiation into antibody-producing cells. Mice with low dietary fibre intake or microbial insufficiency were defective in producing pathogen-specific antibody responses, which could be restored by the intake of SCFAs31. As a further example, the abundance of the genus Roseburia, a butyrate-producer, has been positively correlated with circulating interleukin (IL)-27 levels and T cell responses to an HIV T cell vaccine26.
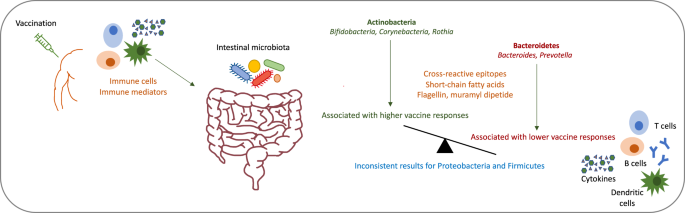
Proposed mechanisms by which vaccination might influence the composition of the intestinal microbiota and by which the intestinal microbiota might influence vaccine responses.
Little is known about the mechanisms by which vaccination might induce changes in the intestinal microbiota. Immune cells and inflammation mediators induced by vaccination, likely migrate with the blood flow from the vaccination side to the intestine, where they interact with microbes (Fig. 1). As an example, it has been hypothesised that vaccination with an HIV T cell vaccine leads to T helper 1 cell stimulation and increased levels of inflammation mediators, which might lead to an increase in the abundance of Roseburia in the intestinal microbiota26. If T cell responses play an important role in inducing changes in the microbiota after vaccination, a further potentially important vaccine which could change the composition of the intestinal microbiota, is Bacillus Calmette-Guérin (BCG) vaccine. BCG is one of the most widely used vaccines globally and it is known to have many non-specific effects.
The limitations of the studies investigating the association between vaccination and changes in the composition of the intestinal microbiota and vice versa include small cohort size; the lack of longitudinal sampling which would allow for better assessment of temporal variations; a large heterogeneity in study design, including different vaccine and vaccine schedules, and timing of collection between vaccination and stool and blood samples. Furthermore, different analysis techniques have been used, and results have been reported on different taxonomic levels. Many studies have used amplicon sequencing which might not be sensitive enough to detect small differences in microbial composition. Moreover, there is a lack of investigations on the mechanisms by which vaccination might induce changes in the intestinal microbiota. This would require not only to investigate the composition of the intestinal microbiota, but also translational approaches, including in vitro and in vivo experiments.
Understanding how vaccines might change the composition of the intestinal microbiota and how these changes could influence vaccine responses, is crucial to optimise vaccine responses, especially in settings or individuals in which/whom lower immunogenicity is expected, such as in developing countries or after antibiotic exposure32. Bacterial species identified to positively influence vaccine responses could be administered as probiotics before vaccination in individuals who are at risk for low vaccine immunogenicity33. Further interventions to possibly optimise the microbiota composition to improve vaccine efficacy include the administration of synbiotics (a mixture of pro- and prebiotics), faecal transplants and small molecules that interact with specific bacterial processes. Importantly, as the composition of the intestinal microbiota has been associated with the risk of many immune- and non-immune-mediated diseases, vaccination could also be used to optimise the composition of the microbiota, for example in infants who are at risk for allergic diseases and asthma34.
References
World Health, O. State of the world's vaccines and immunization (World Health Organization, 2009).
Zimmermann, P. et al. Correlation of vaccine responses. Front. Immunol. 12, 646677 (2021).
Patriarca, P. A., Wright, P. F. & John, T. J. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Rev. Infect. Dis. 13, 926–939 (1991).
Kirkpatrick, B. D. et al. The "performance of rotavirus and oral polio vaccines in developing countries" (PROVIDE) study: description of methods of an interventional study designed to explore complex biologic problems. Am. J. Trop. Med. Hyg. 92, 744–751 (2015).
Levine, M. M. Immunogenicity and efficacy of oral vaccines in developing countries: lessons from a live cholera vaccine. BMC Biol. 8, 129 (2010).
Zimmermann, P. & Curtis, N. Factors that influence the immune response to vaccination. Clin. Microbiol. Rev. 32, e00084–18 (2019).
Zheng, D., Liwinski, T. & Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 30, 492–506 (2020).
Zimmermann, P. & Curtis, N. The influence of the intestinal microbiome on vaccine responses. Vaccine 36, 4433–4439 (2018).
Jordan, A., Carding, S. R. & Hall, L. J. The early-life gut microbiome and vaccine efficacy. Lancet Microbe 3, e787–e794 (2022).
Lynn, D. J. et al. Modulation of immune responses to vaccination by the microbiota: implications and potential mechanisms. Nat. Rev. Immunol. 22, 33–46 (2022).
Huda, M. N. et al. Stool microbiota and vaccine responses of infants. Pediatrics 134, e362–e372 (2014).
Eloe-Fadrosh, E. A. et al. Impact of oral typhoid vaccination on the human gut microbiota and correlations with S. Typhi-specific immunological responses. PLoS ONE 8, e62026 (2013).
Mullie, C. et al. Increased poliovirus-specific intestinal antibody response coincides with promotion of Bifidobacterium longum-infantis and Bifidobacterium breve in infants: a randomized, double-blind, placebo-controlled trial. Pediatr. Res. 56, 791–795 (2004).
Harris, V. et al. Rotavirus vaccine response correlates with the infant gut microbiota composition in Pakistan. Gut Microbes 9, 93–101 (2018).
Praharaj, I. et al. Influence of nonpolio enteroviruses and the bacterial gut microbiota on oral poliovirus vaccine response: a study from South India. J. Infect. Dis. 219, 1178–1186 (2019).
Zhao, T. et al. Influence of gut microbiota on mucosal IgA antibody response to the polio vaccine. NPJ Vaccines 5, 47 (2020).
Parker, E. P. K. et al. Influence of the intestinal microbiota on the immunogenicity of oral rotavirus vaccine given to infants in south India. Vaccine 36, 264–272 (2018).
Harris, V. C. et al. Significant correlation between the infant gut microbiome and rotavirus vaccine response in rural Ghana. J. Infect. Dis. 215, 34–41 (2017).
Shortt, N. et al. A feasibility study: association between gut microbiota enterotype and antibody response to seasonal trivalent influenza vaccine in adults. Clin. Transl. Immunol. 7, e1013 (2018).
Ng, S. C. et al. Gut microbiota composition is associated with SARS-CoV-2 vaccine immunogenicity and adverse events. Gut 71, 1106–1116 (2022).
Borgognone, A. et al. Gut microbiome signatures linked to HIV-1 reservoir size and viremia control. Microbiome 10, 59 (2022).
Pastor-Ibáñez, R. et al. Impact of transcriptome and gut microbiome on the response of HIV-1 infected individuals to a dendritic cell-based HIV therapeutic vaccine. Vaccines 9, 694 (2021).
Guevarra, R. B. et al. Oral vaccination against Lawsoniaintracellularis changes the intestinal microbiome in weaned piglets. Animals 11, 2082 (2021).
Elizaldi, S. R. et al. Rectal microbiome composition correlates with humoral immunity to HIV-1 in vaccinated rhesus macaques. mSphere 4, e00824–19 (2019).
Guo, J. et al. Different immunization methods lead to altered gut flora and varied responses to Mycobacterium tuberculosis infection in mice. J. Infect. Dev. Ctries 14, 1170–1177 (2020).
Borgognone, A. et al. Vaccination with an HIV T-cell immunogen induces alterations in the mouse gut microbiota. npj Biofilms Microbiomes 8, 104 (2023).
Oh, J. Z. et al. TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity 41, 478–492 (2014).
Kim, D. et al. Nod2-mediated recognition of the microbiota is critical for mucosal adjuvant activity of cholera toxin. Nat. Med. 22, 524–530 (2016).
Clasen, S. J. et al. Silent recognition of flagellins from human gut commensal bacteria by Toll-like receptor 5. Sci. Immunol. 8, eabq7001 (2023).
Schaupp, L. et al. Microbiota-induced type I interferons instruct a poised basal state of dendritic cells. Cell 181, 1080–1096.e19 (2020).
Kim, M. et al. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe 20, 202–214 (2016).
Hagan, T. et al. Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell 178, 1313–1328.e13 (2019).
Zimmermann, P. & Curtis, N. The influence of probiotics on vaccine responses - A systematic review. Vaccine 36, 207–213 (2018).
Zimmermann, P. et al. Association between the intestinal microbiota and allergic sensitization, eczema, and asthma: a systematic review. J. Allergy Clin. Immunol. 143, 467–485 (2019).
Acknowledgements
P.Z. is supported by grants from the Swiss National Foundation (PZPGP3_193140), the University of Fribourg and the Fribourg Hospital, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher's note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Zimmermann, P. The immunological interplay between vaccination and the intestinal microbiota. npj Vaccines 8, 24 (2023). https://doi.org/10.1038/s41541-023-00627-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-023-00627-9

Comments
Post a Comment